Lots of interesting abstracts and cases were submitted for TCTAP 2024. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-027
Efficacy of Bioresorbable Scaffolds in Percutaneous Coronary Intervention for Chronic Total Occlusion - A 6-Year Follow-Up Analysis
By Dace Sondore, Ieva Briede, Matiss Linde, Karlis Trusinskis, Inga Narbute, Sanda Jegere, Aigars Lismanis, Indulis Kumsars, Karlis Grikis, Andrejs Erglis
Presenter
Dace Sondore
Authors
Dace Sondore1, Ieva Briede1, Matiss Linde1, Karlis Trusinskis1, Inga Narbute1, Sanda Jegere1, Aigars Lismanis1, Indulis Kumsars2, Karlis Grikis1, Andrejs Erglis1
Affiliation
Pauls Stradins Clinical University Hospital, Latvia1, Pstradins University Hospital, Latvia2
View Study Report
TCTAP A-027
CTO
Efficacy of Bioresorbable Scaffolds in Percutaneous Coronary Intervention for Chronic Total Occlusion - A 6-Year Follow-Up Analysis
Dace Sondore1, Ieva Briede1, Matiss Linde1, Karlis Trusinskis1, Inga Narbute1, Sanda Jegere1, Aigars Lismanis1, Indulis Kumsars2, Karlis Grikis1, Andrejs Erglis1
Pauls Stradins Clinical University Hospital, Latvia1, Pstradins University Hospital, Latvia2
Background
Chronic total occlusion (CTO) and percutaneous coronary intervention (PCI) is associated with longer stent length. This study aimed to evaluate the safety and efficacy of a bioresorbable scaffolds (BRS) in treating CTO to avoid a full metal jacket.
Methods
We conducted a single-center prospective longitudinal case study including 34 patients who underwent PCI of CTO between March 2016 and November 2018. A total of 23 Absorb and 11 Magmaris scaffolds were implanted with additional 33 drug-eluting stents (DES). Quantitative coronary angiography (Medis QAngio XA 7.3) was performed at both the index procedure and a 6-year follow-up, with statistical analysis conducted using IBM SPSS 28.
Results
A total of 76.5% (n = 26) males and 23.5% (n = 8) females were included in the study with mean age of 60.6 ± 9.5 years. Target CTO segment in RCA was 73.5% (n = 25), LAD 23.5% (n = 8) and LCX 2.9% (n = 1). One Absorb BRS per lesion was implanted in 61.8% (n = 21), two Absorb BRS in 5.9% (n = 2) and one Magmaris in 32.4% (n = 11) of patients were implanted with additional DES. The mean length of occlusion was 24.8 ± 14.6 mm and total mean length of BRS/DES was 49.6 ± 20.4 mm. During the follow-up there was a statistical increase in mean residual diameter stenosis (22.9% - 33.5%, p < 0.01), mean residual area stenosis (39.1% - 53.6%, p < 0.01) and residual length of stenosis (5.2% - 7.1%, p = 0.04). 26.5% (n = 9) of patients had target lesion failure (TLF). Median time to TLF was 74 (95% CI, 68.3 - 79.7) months. 96.7% (n = 30) of patients still had a patent treated CTO artery and one patient had reocclusion by the time of follow up.
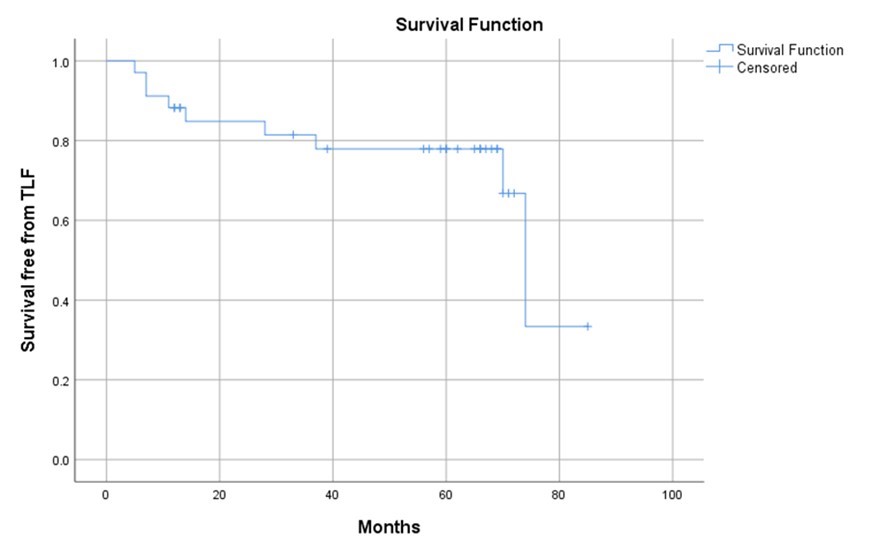

Conclusion
This study demonstrates a notable success rate in maintaining patent CTO cases through the use of BRS during long-term follow-up. BRS has proven to be a safe and feasible option for PCI of CTOs, enabling us to avoid long segment stenting.

