Lots of interesting abstracts and cases were submitted for TCTAP 2023. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-079
AI-FFR: Add Innovation to FFR Value Measurements and Present New Way
By Kyungrae Hwang
Presenter
Kyungrae Hwang
Authors
Kyungrae Hwang1
Affiliation
CG BIO, Korea (Republic of)1
View Study Report
TCTAP A-079
Innovative Devices and Futuristic Therapies
AI-FFR: Add Innovation to FFR Value Measurements and Present New Way
Kyungrae Hwang1
CG BIO, Korea (Republic of)1
Background
FFR-guided PCI is an important PCI treatment method that has been implemented worldwide as it is applied in ESC/ACC guidelines as an important guideline of PCI treatment through many clinical trials. And products for more diverse FFR measurements continue to be developed. However, the preparation process and proficiency are still required to measure FFR, and some patients have limitations such as discomfort and adenosine resistance. In order to overcome them, methods of measuring FFR with only Angiograms are coming out in several companies, and clinical data are being published. FFRxa is also a software program that presents FFR values based on Angiograms, and the difference is that there is a key point in speed and accuracy to overcome some handicaps of existing Angio based FFR.
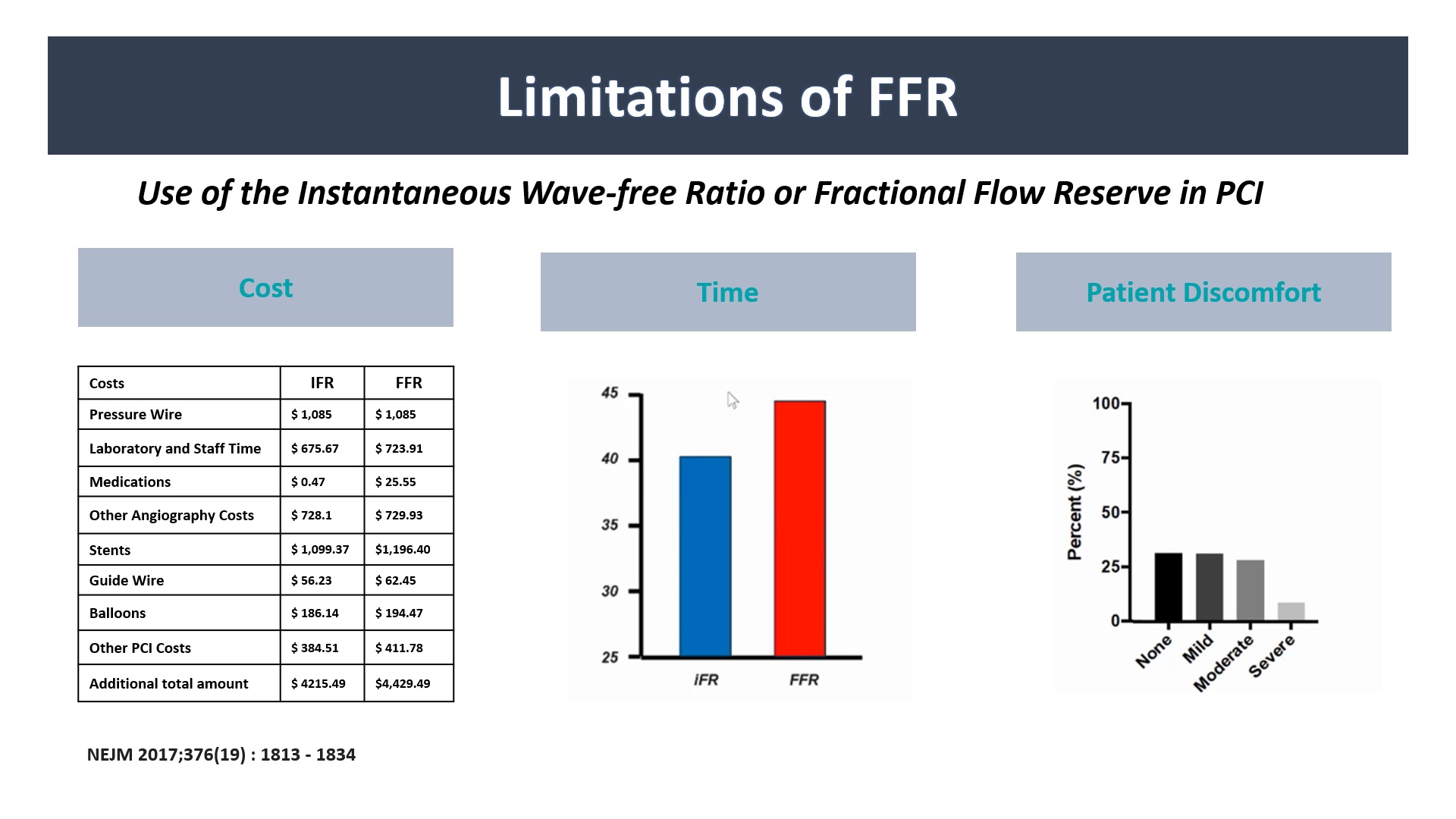



Methods
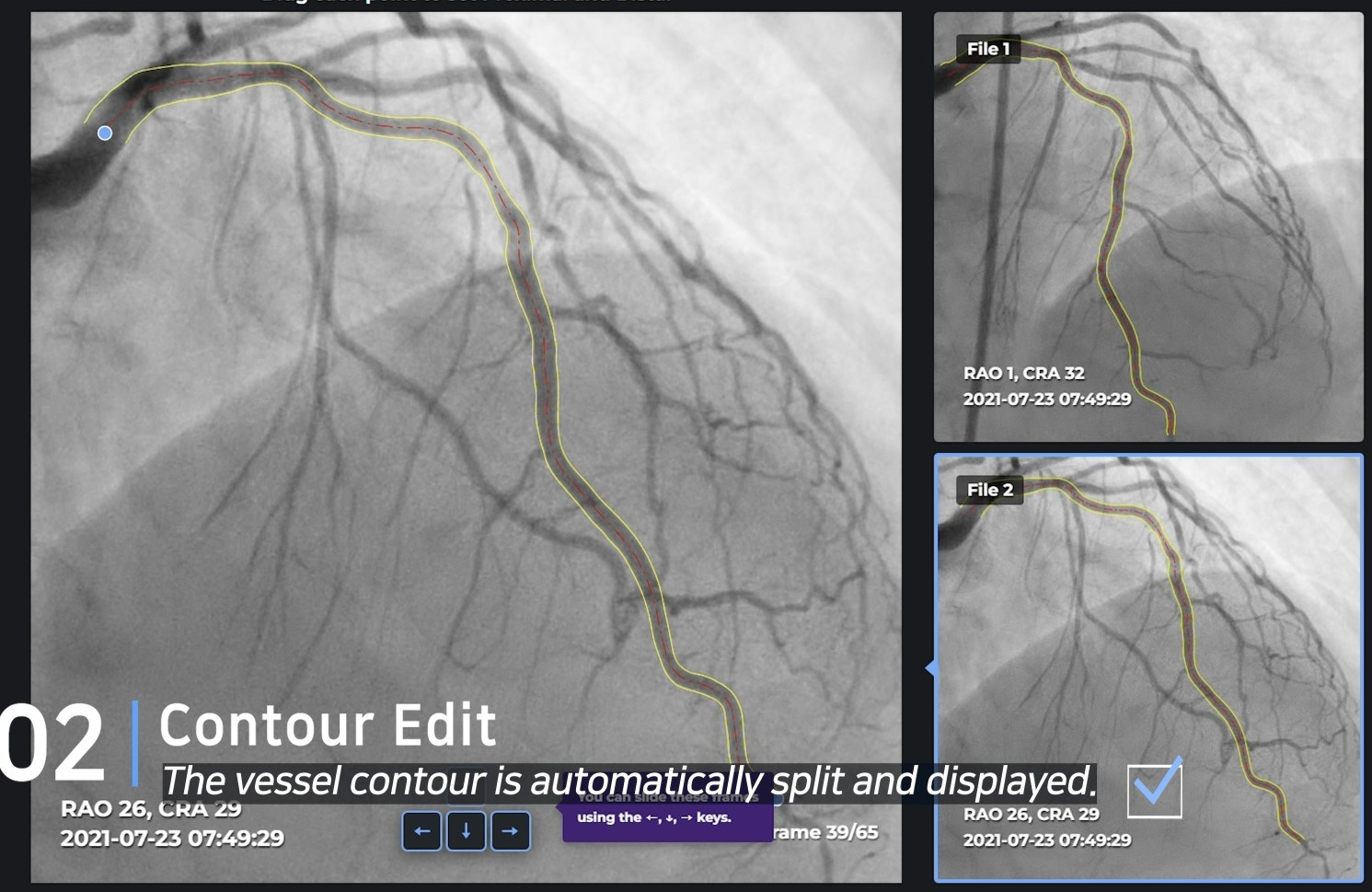
Basically, the FFR value is measured through a 3D configuration implemented at an angle of 30 degrees or more with two angiograms. When the necessary CIP(Common Image Point) is found through the selected frames, and the location of the blood flow is determined by the TIMI frame, the FFR value is accurately realized.
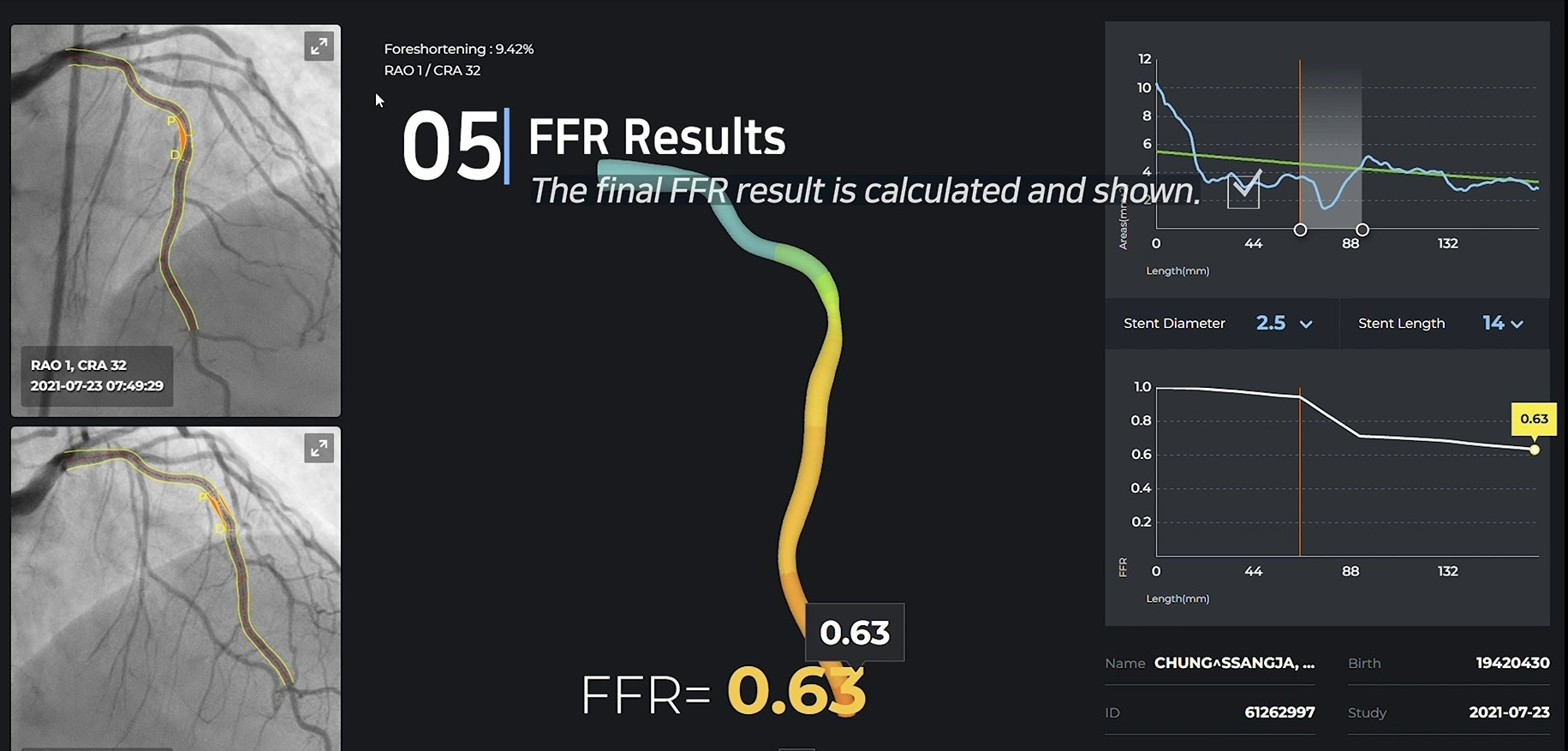
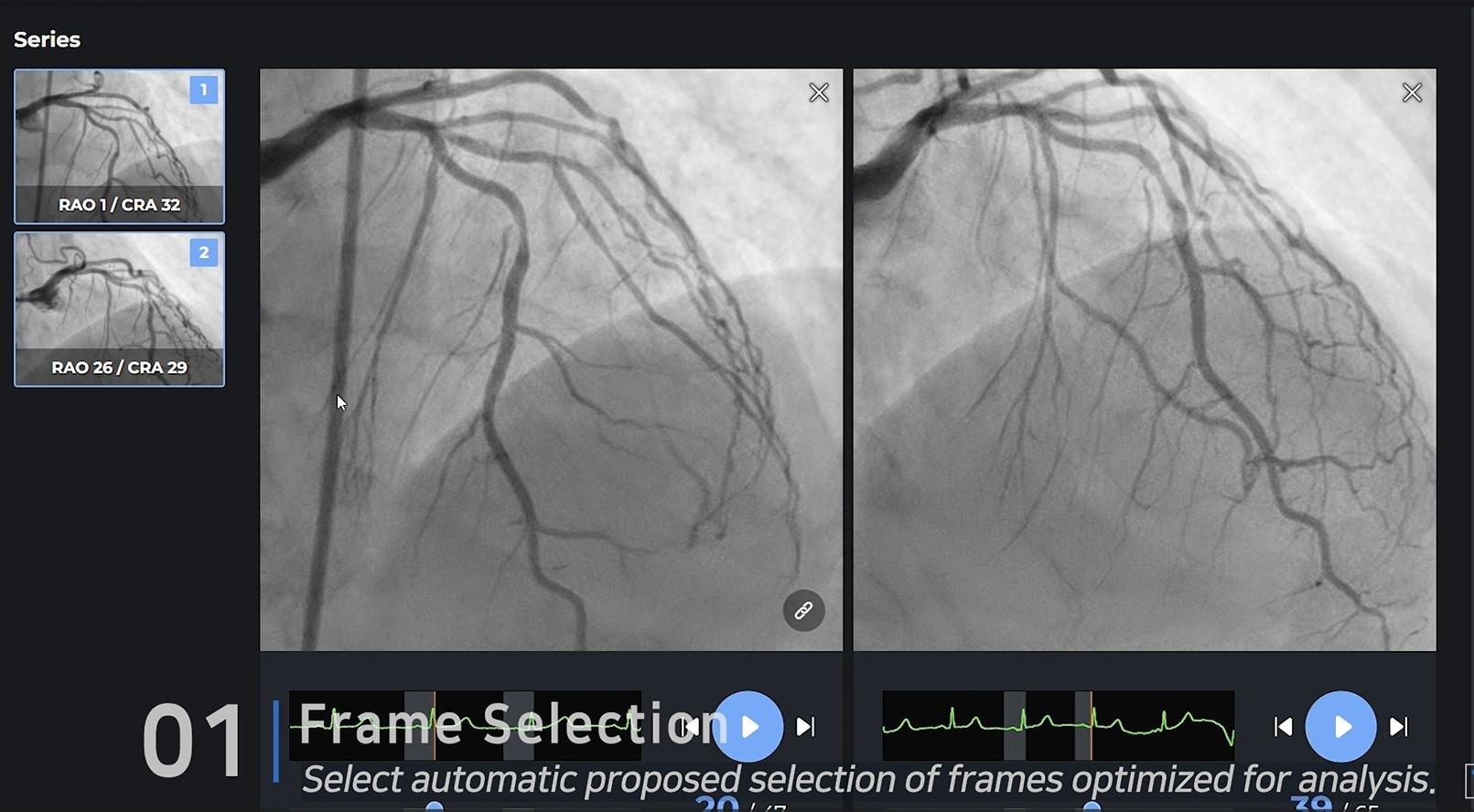
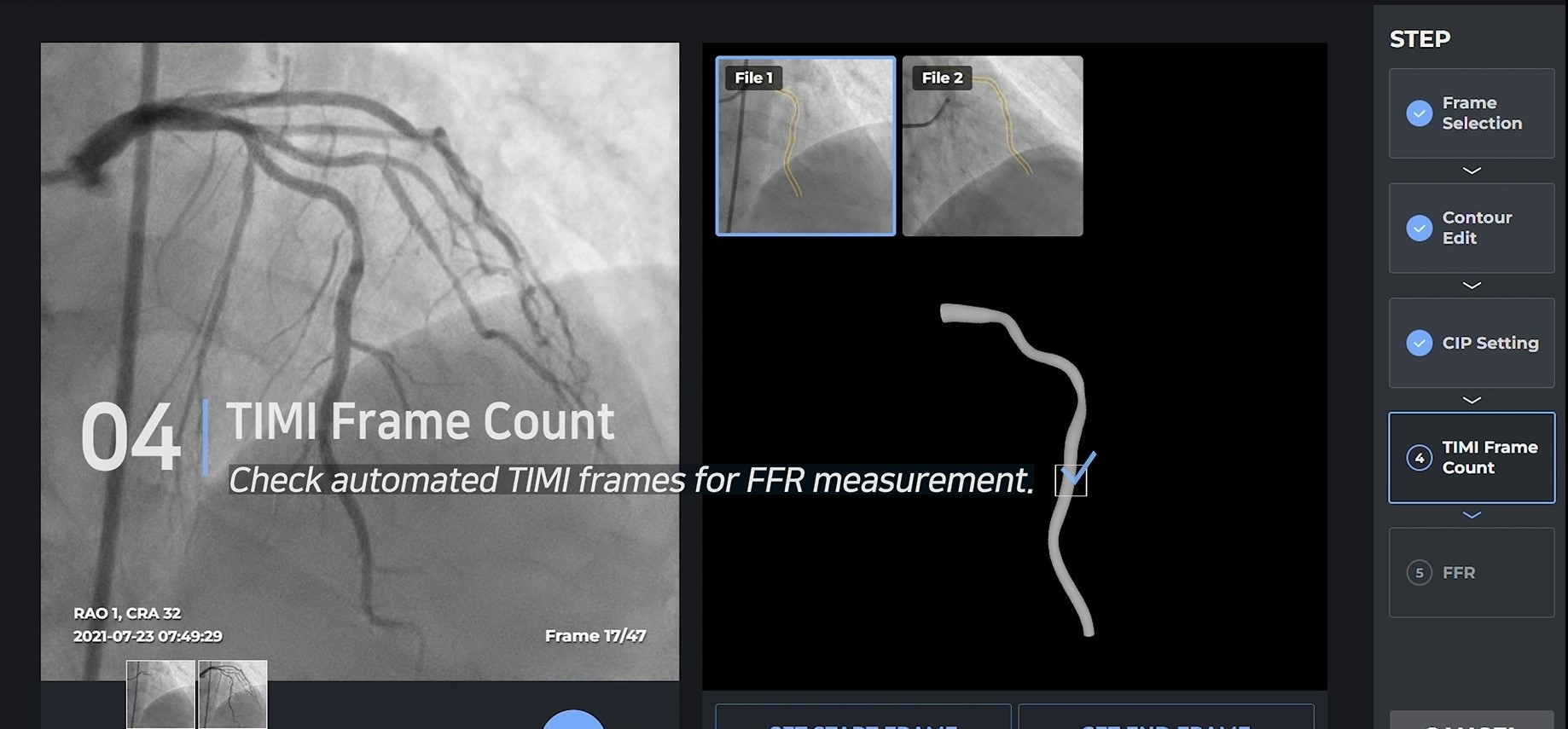



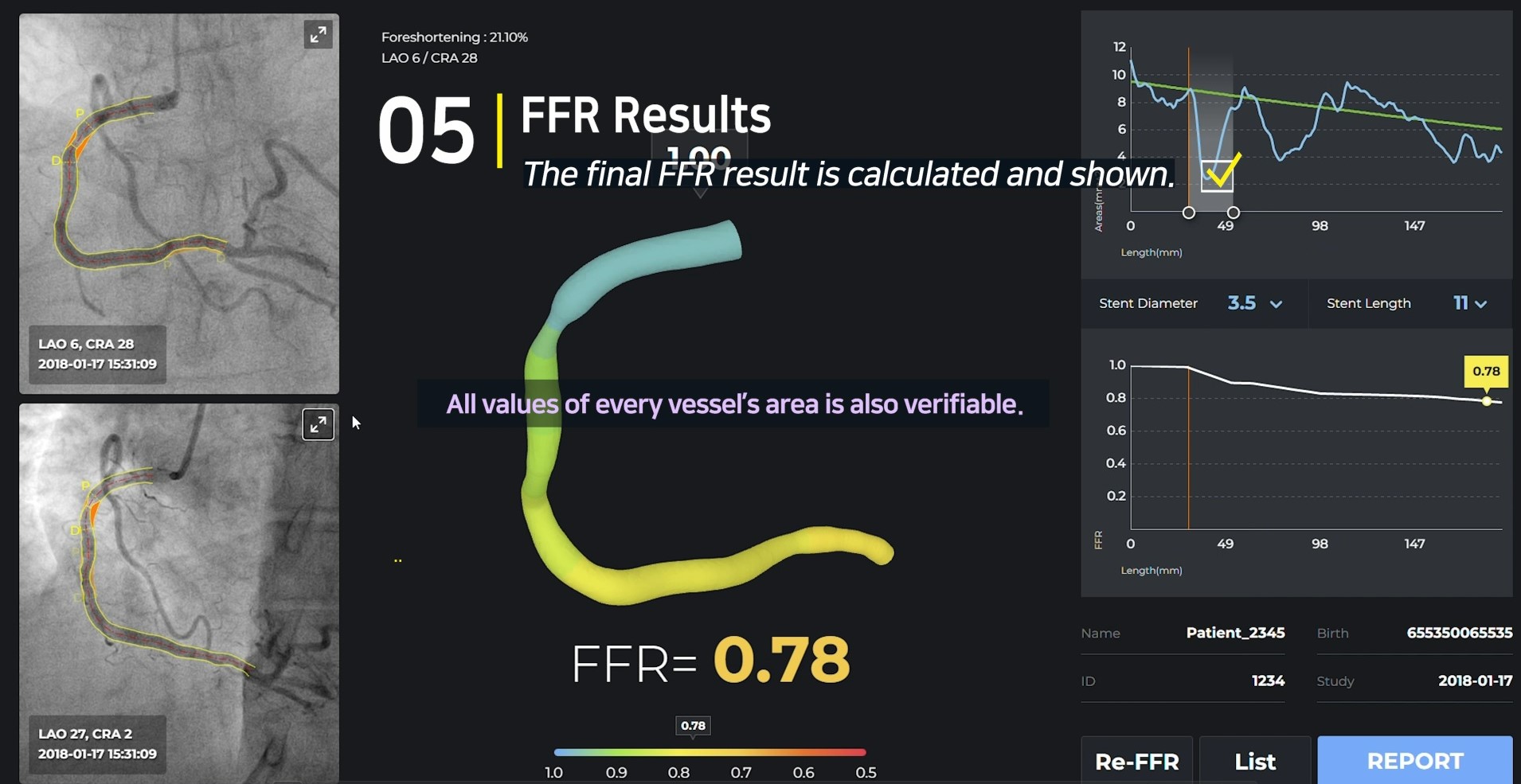
Results
Unlike most other companies making manual measurements, two of the four main processes have been automated, and the other two processes, CIP and TIMI Frame selection, are also automated through clinical trials. Currently, 10 centers in Korea are undergoing clinical trials for KFDA licenses, and data verified by Pilot will also be released in January, so it will be available during the conference.
MPFFRxa demonstration(221024).mp4
MPFFRxa demonstration(221024).mp4
Conclusion
FFRxa, which measures FFR based on two Angiograms, the most important diagnostic method, proves to be the fastest and most accurate than any products currently available. Looking at the changes in FFR's new paradigm, we believe that it will be of great support to all medical staffs to perform the PCI procedure more conveniently.


