Lots of interesting abstracts and cases were submitted for TCTAP & AP VALVES 2020 Virtual. Below are accepted ones after thoroughly reviewed by our official reviewers. Don¡¯t miss the opportunity to explore your knowledge and interact with authors as well as virtual participants by sharing your opinion!
* The E-Science Station is well-optimized for PC.
We highly recommend you use a desktop computer or laptop to browse E-posters.
ABS20200408_0003
| Pharmacology/Pharmacotherapy | |
| Influence of Individual Proton Pump Inhibitors on Clinical Outcomes in Patients Receiving Clopidogrel following Percutaneous Coronary Intervention: A Systematic Review with Meta-Analysis | |
| Dong Young Lee1, Je Sang Kim2, Seung Yong Shin3 | |
| The Jain Hospital, Korea (Republic of)1, Bucheon Sejong General Hospital, Korea (Republic of)2, Korea University Ansan Hospital, Korea (Republic of)3 | |
|
Background:
One of the concerns surrounding the use of proton-pump inhibitors (PPI) is a potential interaction with the antiplatelet agent clopidogrel, a prodrug requiring activation by cytochrome P450isoenzymes, and the literature consists of contrasting findings on this issue.1,2 PPI is competitive inhibitors of the cytochrome P450 pathway. Moreover, individual PPI has a different effect on CYP metabolism and thus may have adverse effects on clopidogrel metabolism and subsequent cardiovascular outcomes to various degrees. Compared with numerous studies showing the interaction of overall PPI with clopidogrel, meta-analyses for the association of clopidogrel and individual PPI in patients on dual antiplatelet agents (DAPT) was scarce. Recently, many new studies were published based on the cardiovascular outcomes observed in patients treated with clopidogrel plus PPI. Therefore, this study aimed to evaluate the association of concomitant use of clopidogrel with individual PPIs in patients on dual antiplatelet by a systematic review, meta-analysis.
|
|
|
Methods:
MEDLINE(through PubMed), EMBASE, Cochrane Central Register of Controlled Trials(CENTRAL) and relevant websites were searched for pertinent published or unpublished studies using keyword associated with clopidogrel and proton-pump inhibitors (from inception to March 22, 2020) by two independent evaluators (D.Y.L. and J.S.K.). Search terms used were combined with the Boolean operator ¡®AND¡¯ and ¡®OR¡¯, The following key search terms were used: (proton pump inhibitor OR PPI OR omeprazole OR pantoprazole OR lansoprazole OR esomeprazole OR rabeprazole OR dexlansoprazole) AND (dual antiplatelet therapy OR dual anti-platelet therapy OR DAPT OR DAT OR thienopyridine OR P2Y12inhibitor OR clopidogrel). Another search was carried out through the references cited in various studies, and the electronic search strategy was complemented by manual examination of references cited by included articles, recent reviews, editorials, and meta-analyses. No restriction was imposed on language, study period, or sample size.
|
|
|
Results:
The 5 RCTs with 4,503 patients and the 21 observational studies with 164,951 patients were abstracted. The pooled meta-analysis of 26 studies exhibited a higher risk of MACE in patients with any PPI (RR, 1.24; 95% CI, 1.07-1.43; I2=91.0%) in a random effect meta-analysis. Stratifying by RCT and observational studies, the risk of MACE inpatients receiving any PPI and clopidogrel were significantly increased in a pooled analysis of 21 observational studies with random effect model. But in the 5 RCTs , there was no significant increased risk of MACE (observational studies: RR = 1.27; 95% CI = 1.09-1.48; I2 = 92%; RCTs: RR = 1.01; 95% CI =0.76-1.35; I2 = 0). In case of analysis with individual PPIs, random-effects analyses of the 26 studies revealed an increased risk for MACEs for those taking pantoprazole (RR 1.28; 95% CI 1.06–1.55), lansoprazole (RR 1.36;95% CI 1.20–1.54) compared with patients on no PPI. This association was not significant for omeprazole (RR 0.94; 95% CI 0.62–1.41), esomeprazole (RR 1.14;95% CI 0.75–1.72) and rabeprazole (RR 1.35; 95% CI 0.60–3.04). For further robustness of the results, we repeated the entire analysis including the study reporting adjusted HRs only. The increased risk of MACEs was similar in four classes of PPIs, but rabeprazole (HR: 1.32; 95% CI: 0.69-2.53) wasn't.
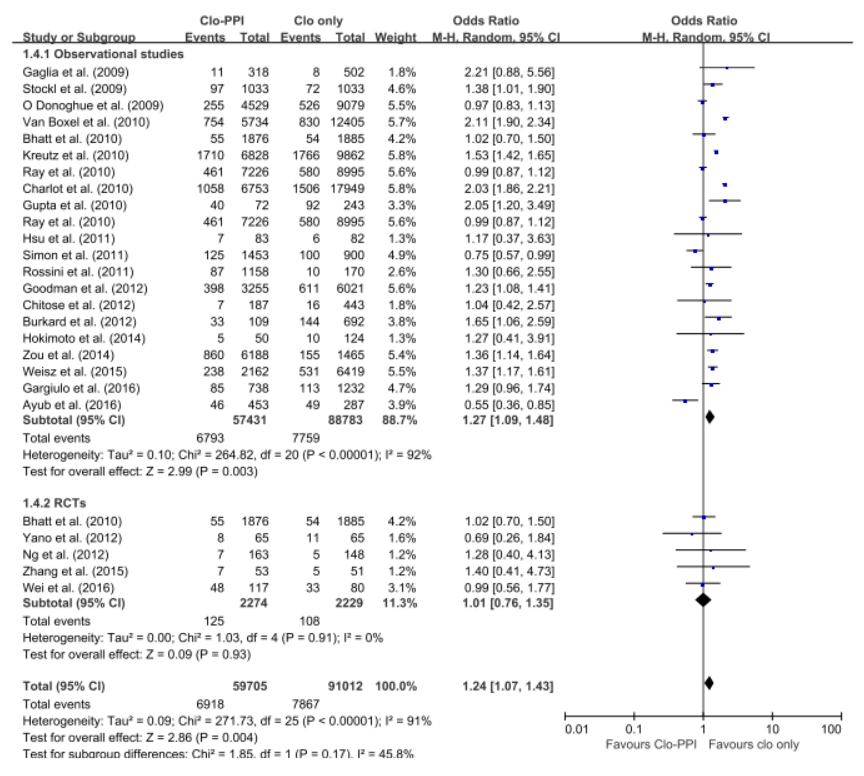 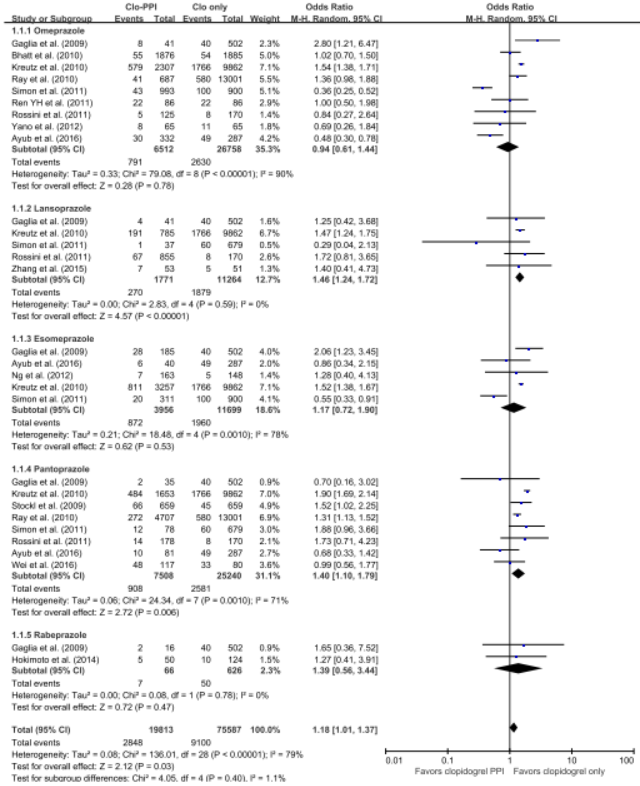 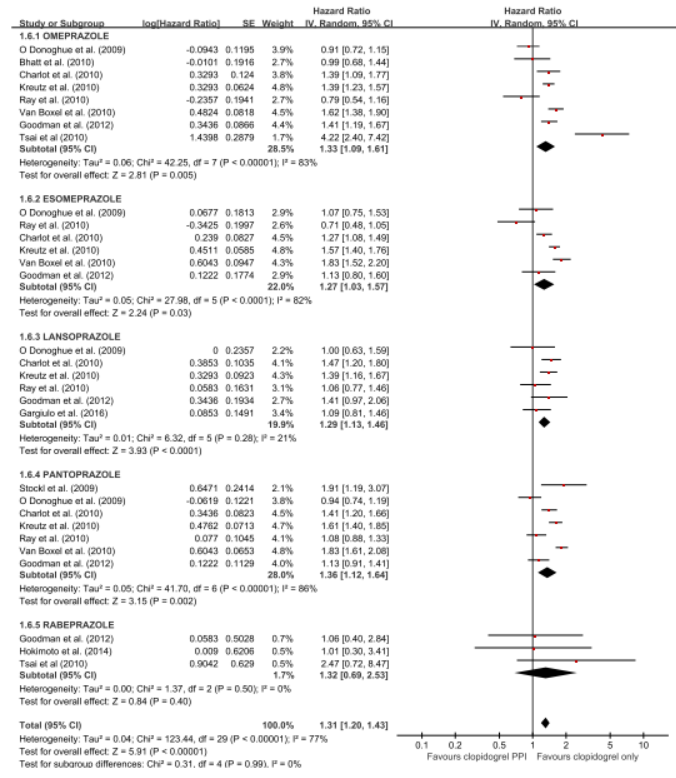 |
|
|
Conclusion:
The pooled meta-analysis of 26 studies exhibited a higher risk of MACE in patients with any PPI (RR, 1.24; 95% CI, 1.07-1.43; I2=91.0%) in a random effect meta-analysis. In case of analysis with individual PPIs, random-effects analyses of the 26 studies revealed an increased risk for MACEs for those taking pantoprazole (RR 1.28; 95% CI 1.06–1.55), lansoprazole (RR 1.36;95% CI 1.20–1.54) compared with patients on no PPI. This association was not significant for omeprazole (RR 0.94; 95% CI 0.62–1.41), esomeprazole (RR 1.14;95% CI 0.75–1.72) and rabeprazole (RR 1.35; 95% CI 0.60–3.04). For further robustness of the results, we repeated the entire analysis including the study reporting adjusted HRs only. The increased risk of MACEs was similar in four classes of PPIs, but rabeprazole (HR: 1.32; 95% CI: 0.69-2.53) wasn't.
|
|