Lots of interesting abstracts and cases were submitted for TCTAP & AP VALVES 2020 Virtual. Below are accepted ones after thoroughly reviewed by our official reviewers. Don¡¯t miss the opportunity to explore your knowledge and interact with authors as well as virtual participants by sharing your opinion!
* The E-Science Station is well-optimized for PC.
We highly recommend you use a desktop computer or laptop to browse E-posters.
ABS20191116_0002
| Innovative Devices and Futuristic Therapies | |
| Novel Dedicated Paravalvular Leak Occluder Device: Design and Preclinical Evaluation | |
| Grzegorz Smolka1, Michal Tendera1, Dariusz Dudek2, Emil Plowiecki3, Urszula Paslawska4, Wojciech Zasada5, Wojciech Wojakowski1 | |
| Medical University of Silesia, Poland1, Jagiellonian University Medical College, Poland2, Balton Poland, Poland3, Wroclaw University of Veterinary Sciences, Poland4, KCRI, Poland5 | |
|
Background:
Paravalvular leak (PVL) is a complication of surgical valve replacement which leads to heart failure and hemolytic anemia and warrants treatment in approximately 5% of patients. Transcatheter closure of PVL is an accepted method of treatment provided complete sealing can be achieved. Most frequently used transcatheter devices are either vascular plugs (AVP II, AVP III), or dedicated PLD devices. However currently available devices are not optimized for closure of all PVL due to heterogeneity of the shapes and sizes. Current devices have limited number of sizes and shapes and vascular plugs do not have the sealing fabric. The dedicated PVL device optimized for PVL closure is needed. We designed novel PVL device and tested it in the preclinical model.
|
|
|
Methods:
Device design was based on the clinical registry in which distribution of sizes and anatomies was evaluated in 220 patients with mitral and aortic PVL treated in our referral centre. A prospective clinical trial validating the novel PVL sizing balloon was carried out. The device was designed based on the computational model of PVL sizes and shapes obtained from angio-CT and 3D TOE. Nitinol device produced by Balton Poland consists of three discs with three layers of PTFE sealing fabric. The disc sizes range from 6-16 mm and device length 5-10mm. Tapered and braided delivery sheath was designed. The bench tests were carried out using 3D printed models of PVL. Project was funded by National Centre for Research and Development (STRATEGMED2/269488/7/NCBR/2015)
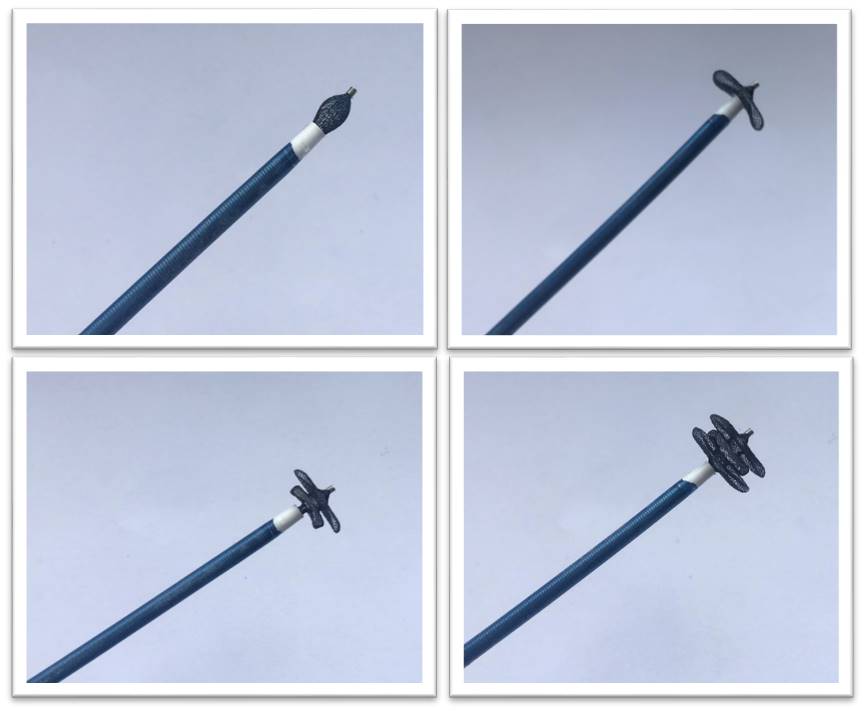 |
|
|
Results:
Preclinical animal study was done in pigs with device implanted into abdominal aorta. Histopathology study was done acutely (24 hrs), and after 14 days and 28 days showing biocompatibility and low inflammation. No thrombus formation was observed.
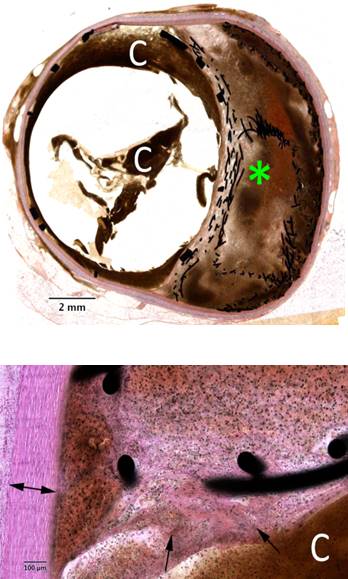 |
|
|
Conclusion:
We designed novel dedicated PVL device with triple layer of sealing material, controlled orientation and deployment, multiple sizes to address majority of anatomies and possibility of patient-tailored production. The device is FIM-ready (Q1 2020).
|
|