Lots of interesting abstracts and cases were submitted for TCTAP & AP VALVES 2020 Virtual. Below are accepted ones after thoroughly reviewed by our official reviewers. Don¡¯t miss the opportunity to explore your knowledge and interact with authors as well as virtual participants by sharing your opinion!
* The E-Science Station is well-optimized for PC.
We highly recommend you use a desktop computer or laptop to browse E-posters.
ABS20190928_0002
| Acute Coronary Syndromes (STEMI, NSTE-ACS) | |
| Impact of Trimetazidine on Infarct Size After Primary Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction | |
| Geng Qian1, Qinhua Jin1 | |
| Chinese PLA General Hospital, China1 | |
|
Background:
In this double blind randomized placebo controlled parallel-group trial, we investigated the effectso f trimetazidine for the reduction of infarct size in patients undergoing revascularization for ST-elevation myocardial infarction (STEMI).
|
|
|
Methods:
Patients presenting with STEMI within 12 h of the onset of pain randomly received trimetazidine (n=87) or placebo (n= 86) in a double-blind manner. Trimetazidine treatment was commenced before primary PCI and maintained for 6 months after the procedure. The primary endpoint was infarct size measures by cardiac magnetic resonance (CMR) in 148 subjects at 6–8 days after primary PCI. This trial is registered with www.clinicaltrials.gov, number NCT02826616.
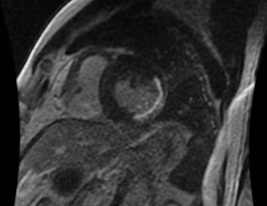 |
|
|
Results:
Trimetazidine group and control group were well-matched in baseline characteristics. Trimetazidine reduced infarct compared with the infarct size of control subjects (22.1¡¾11.8% [n =74] vs. 26.9¡¾11.9% [n=74]; p=0.010, 28¡¾18g [n =74] vs. 35¡¾19g [n=74]; p=0.022). Trimetazidine also reduced the myocardial microvascular obstruction (MVO) measured CMR (29.7% [22/7] vs. 52.7% [39/74]; p=0.007).
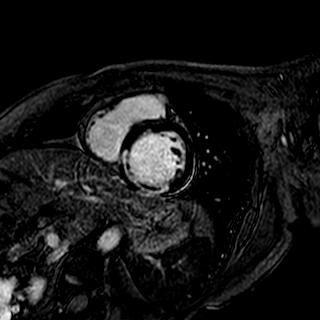 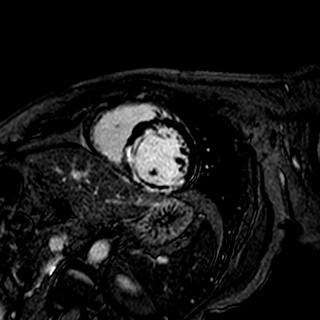 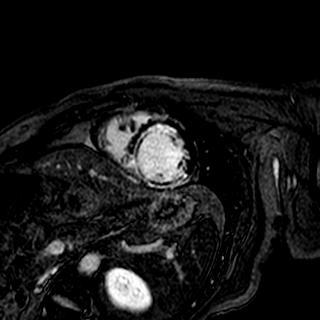 |
|
|
Conclusion:
This randomized study demonstrated that in STEMI treated by primary PCI, trime tazidine, initiated prior to primary PCI, reduced MI size, increased myocardial salvage, and reduced myocardial MVO.
|
|