Lots of interesting abstracts and cases were submitted for TCTAP 2023. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP C-183
Device-Related Hemolytic Anemia After Transcatheter Closure of a Ruptured Ventricular Septum: To Wait or to Act?
By Ingrid Maria Pardede, Sunanto Ng, Radityo Prakoso
Presenter
Ingrid Maria Pardede
Authors
Ingrid Maria Pardede1, Sunanto Ng2, Radityo Prakoso3
Affiliation
Pelita Harapan University, Indonesia1, Universitas Pelita Harapan, Indonesia2, National Cardiovascular Center Harapan Kita, Indonesia, Indonesia3,
View Study Report
TCTAP C-183
STRUCTURAL HEART DISEASE - Others (Structural Heart Disease)
Device-Related Hemolytic Anemia After Transcatheter Closure of a Ruptured Ventricular Septum: To Wait or to Act?
Ingrid Maria Pardede1, Sunanto Ng2, Radityo Prakoso3
Pelita Harapan University, Indonesia1, Universitas Pelita Harapan, Indonesia2, National Cardiovascular Center Harapan Kita, Indonesia, Indonesia3,
Clinical Information
Patient initials or Identifier Number
SHLV01024761
Relevant Clinical History and Physical Exam
A 60-year-old male was referred with worsening heart failure post extensive anterior ST-elevation myocardial infarction 2 months prior to admission. He was previously treated conservatively. Cardiopulmonary examination was significant for grade 4/6 holosystolic murmur and reduced vesicular breath sounds on the right hemithorax with no crackles. Bilateral pitting edema was seen on the lower extremities.
Relevant Test Results Prior to Catheterization
The 12-lead electrocardiogram showed pathologic Q waves in the anterior precordial leads. Trans-thoracic echocardiogram revealed a 8 x13 mm muscular ventricular septal rupture with left-to-right shunt. Computed tomography revealed an oval shaped muscular ventricular rupture size 9 x 13 mm with optimal apical rim.
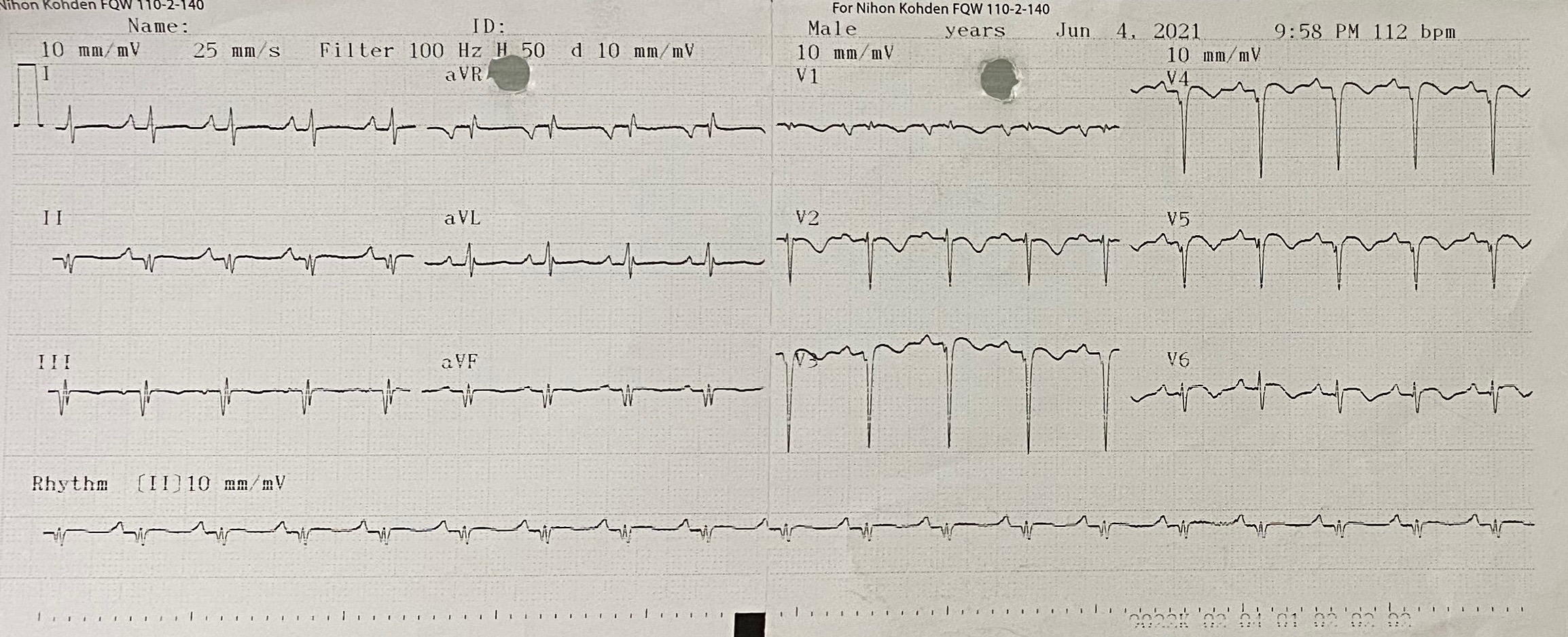
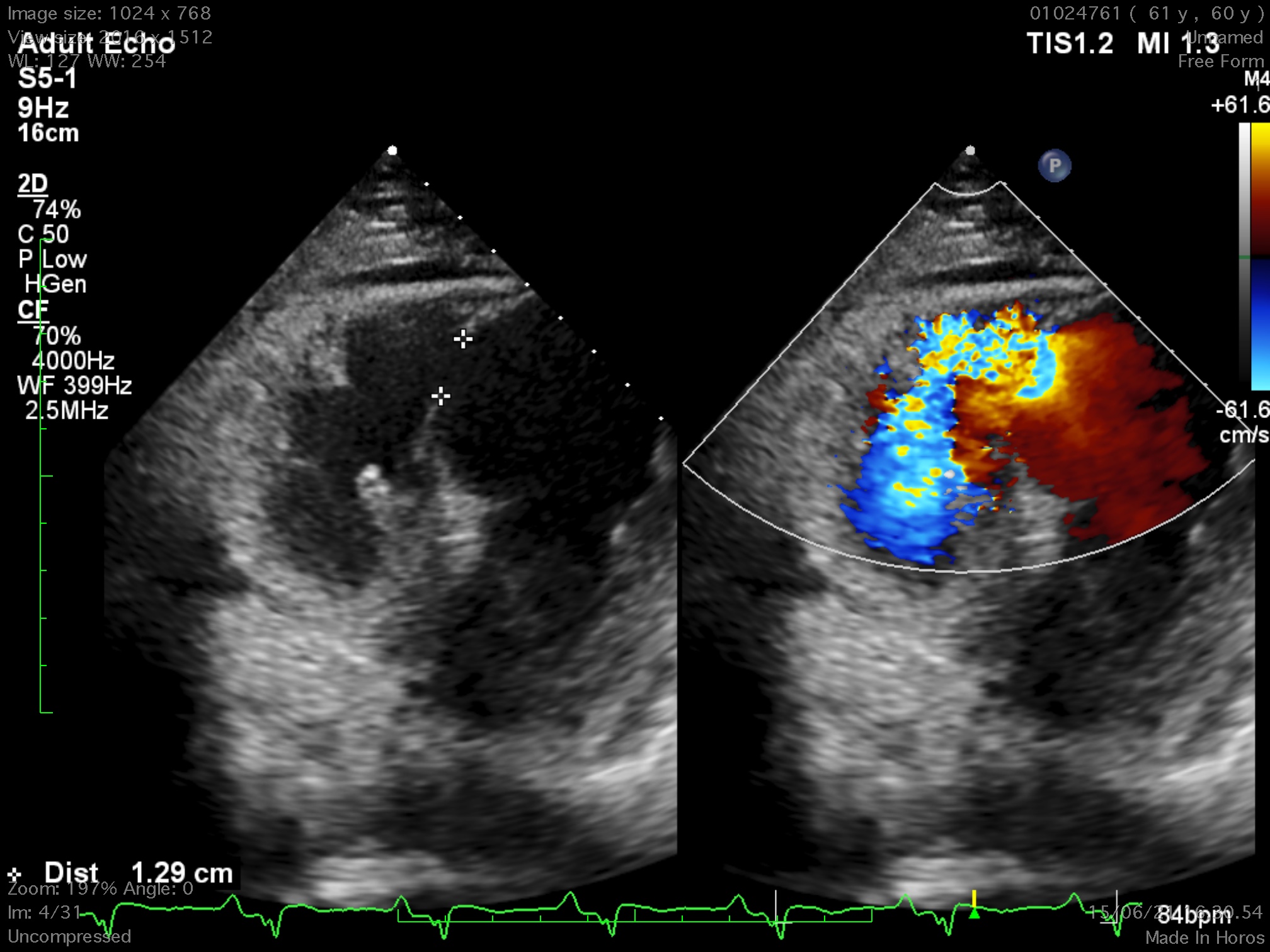




Relevant Catheterization Findings
Coronary angiography revealed total occlusion at mid segment of left anterior descending and normal other coronary arteries. It was decided to not doing intervention at the occluded artery because of non-viability segment.
Interventional Management
Procedural Step
TEE-guided transcatheter occlusion procedure with antegrade technique was performed. Cera ASD occluder no.18 device (LifeTech Scientific, China) was implanted without procedural complications. A few hours later, he experienced gross hematuria and progressive jaundice. Bloods showed anemia with increased reticulocyte count, total and indirect bilirubin and creatine kinase. Hematuria persisted during several days. Investigations to exclude other causes of hemolytic anemia included hemoglobin electrophoresis, Coombs’ test and G6PD were all normal. A residual shunt on TTE was noted during early observation. A diagnosis of device-related hemolytic anemia was made. Patient was treated conservatively with close monitoring on the development of hematuria and related-clinical situation. Two weeks after the hematuria, the condition getting resolved. Repeated echocardiogram revealed complete occlusion of the ventricular septal device with trivial residual shunt. The patient was well at discharge and has remained without recurring hematuria after 30 days.

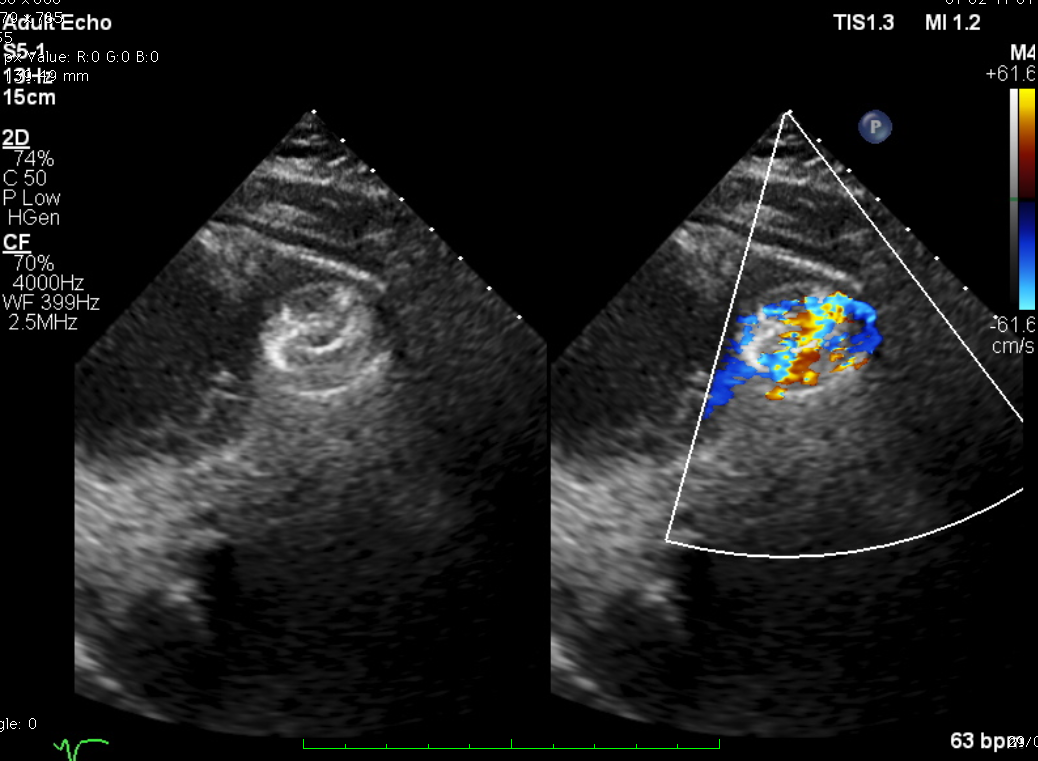
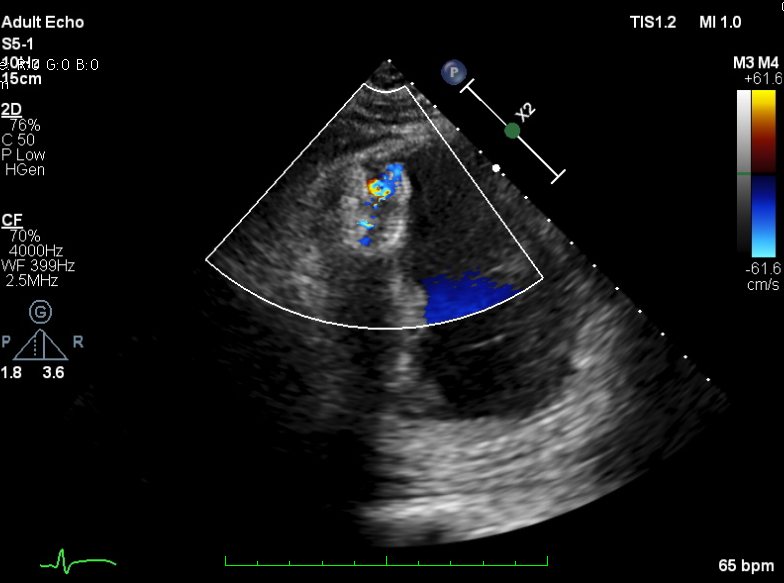



Case Summary
Hemolytic is one of the complication in closure device procedures, in particular in those with residual shunt. Careful consideration should be taken before crossing over to surgical management, where within this period of watchful waiting symptomatic management and observation are essential. The duration of the observation period should be determined by patient response and clinical judgement. In our reported case, we observed the resolution of device-related hemolytic concomitant with complete device occlusion might resolve as long as 30 days.


