Lots of interesting abstracts and cases were submitted for TCTAP 2021 Virtual. Below are accepted ones after thoroughly reviewed by our official reviewers. Don’t miss the opportunity to explore your knowledge and interact with authors as well as virtual participants by sharing your opinion!
TCTAP C-105
Presenter
Quy Nguyen Phu Le
Authors
Quy Le Nguyen Phu1
Affiliation
University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam1,
View Study Report
TCTAP C-105
STRUCTURAL HEART DISEASE - Congenital Heart Disease (ASD, PDA, PFO, VSD)
Progressive Heart Block After Device Occlusion of Simple Atrial Septal Defect: A Case Report
Quy Le Nguyen Phu1
University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam1,
Clinical Information
Patient initials or Identifier Number
18972620
Relevant Clinical History and Physical Exam
A 5-year-old girl was diagnosed incidentally secundum atrial septal defect by echocardiography. No special medical problems were recorded. Clinical examination found normal vital signs with SpO2 98%, heart rate of 100 bpm, blood pressure of 90/55 mmHg, split S2 and systolic murmur at left upper sternal border.
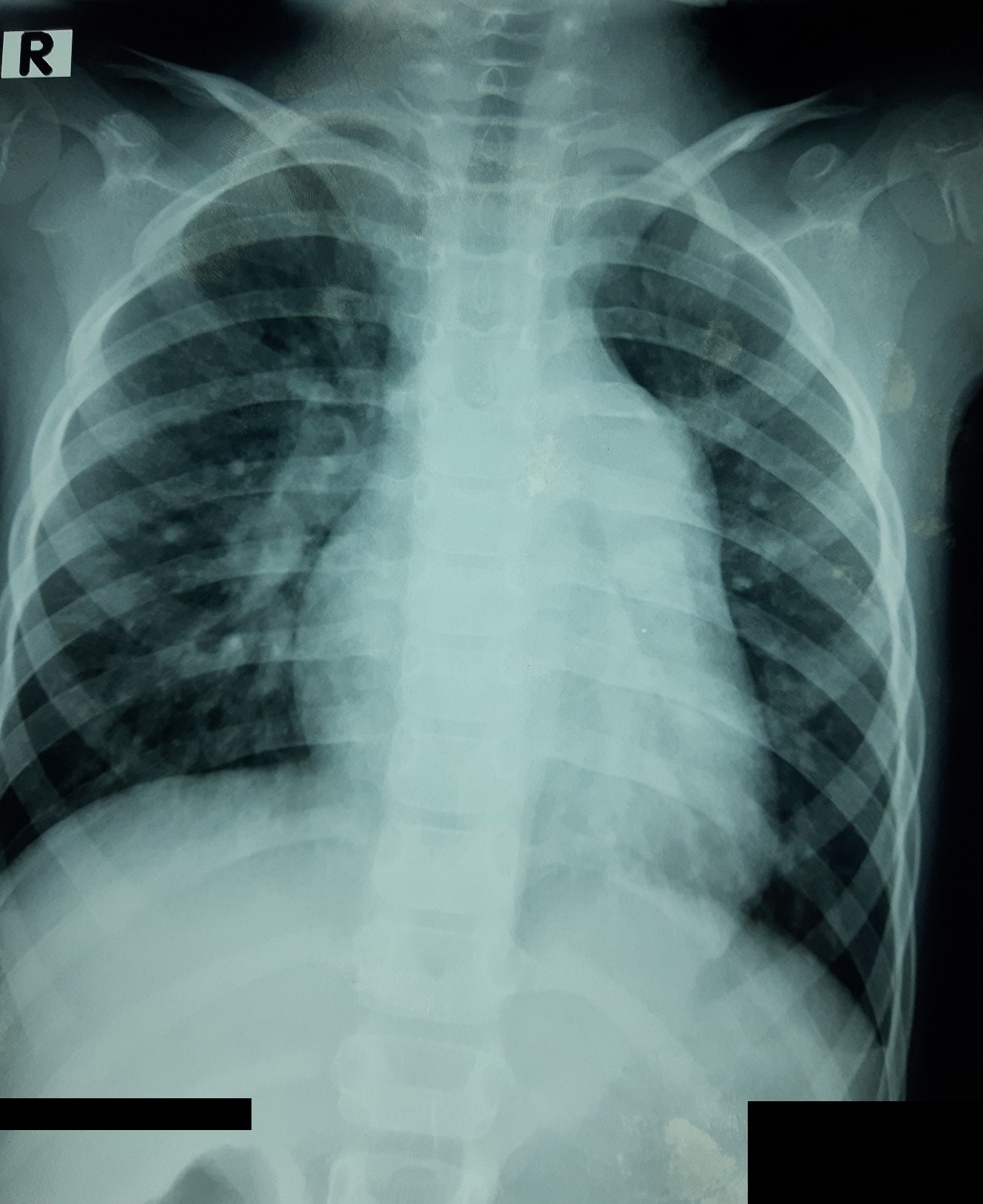

Relevant Test Results Prior to Catheterization
ECG recorded a regular sinus rhythm with HR of 100 bpm, normal AV conduction, right deviation QRS axis, RV enlargement. Transthoracic echocardiography (TTE) showed a secundum ASD 18mm, with the dimension of aortic rim, posterior rim, PV rim, AV rim, SVC rim, IVC rim were 2 mm, 5 mm, 6 mm, 8 mm, 4 mm, 7 mm, respectively. The length of the atrial septum was 34mm. Shunt through ASD was left to right. The RV was dilated, mild TR with PAPs 35 mmHg, no MR, normal pulmonary and systemic venous returns.
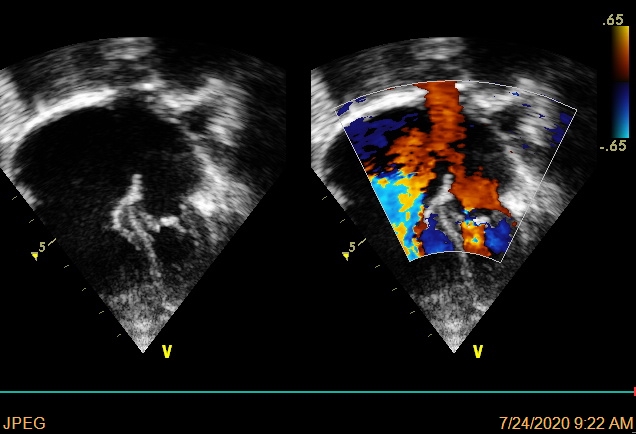


Relevant Catheterization Findings
A 5F introducer sheath was inserted into the right femoral vein. A 4F multipurpose catheter (Merit Medical System, USA) and 0.035’’ Guidewire M (Terumo Corporation, Japan) were advanced through the sheath from the IVC to the left atrium and left upper pulmonary vein. The Guidewire M was exchanged with AmplatzerTM guidewire (AGA Medical Corporation, USA). The 24 mm sizing balloon was inserted to estimate the diameter of atrial septal defect. The stretching diameter of the ASD was 21.5mm.
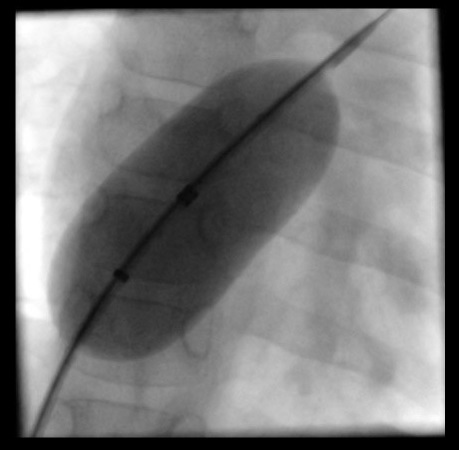

Interventional Management
Procedural Step
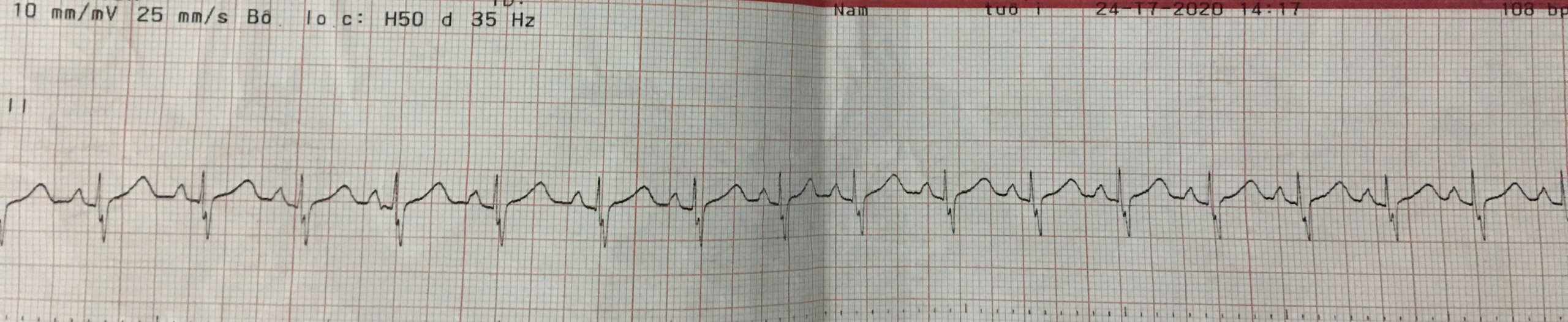
A 9F AmplatzerTM TorqVue 45o Delivery system (Abbott Medical, USA) was advanced from the IVC toward the left upper pulmonary vein. The 22 mm AMPLATZERTM septal occluder (Abbott, USA) device was failed to close the atrial septal defect after three attempts. The 9F AmplatzerTM TorqVue 45o Delivery system was exchanged with the 11F Occlutech Delivery Set (Occlutech GmbH., Germany). The 24 mm Figulla Flex II device (Occlutech GmbH., Germany) was advanced to close atrial septal defect successfully. The device was checked by transthoracic echocardiography with correct position, fixed well to all rims without residual shunt and other complications. Before releasing the device, grade II Mobitz I atrioventricular block was recorded and disappeared after a dose of atropine0.25 mg. We decided to release the device. One day after procedure, complete AV block appeared with stable hemodynamic status. TTE found the device remained fixed well on the atrial septum without compression on adjacent structures, no worsening tricuspid regurgitation. The patient was given 2 mg/kg of prednisone daily for 7 days. However, heart block did not improve. The patient was then undergone open heart surgery to remove the device and closed the atrial septal defect with autologous pericardial patch. Post-operation, the patient gradually recovered her sinus rhythm within 1 week and was discharged from the hospital afterwards.
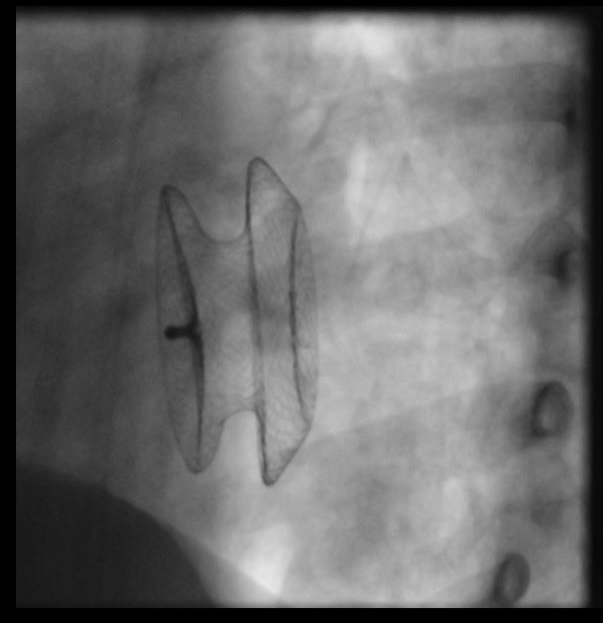
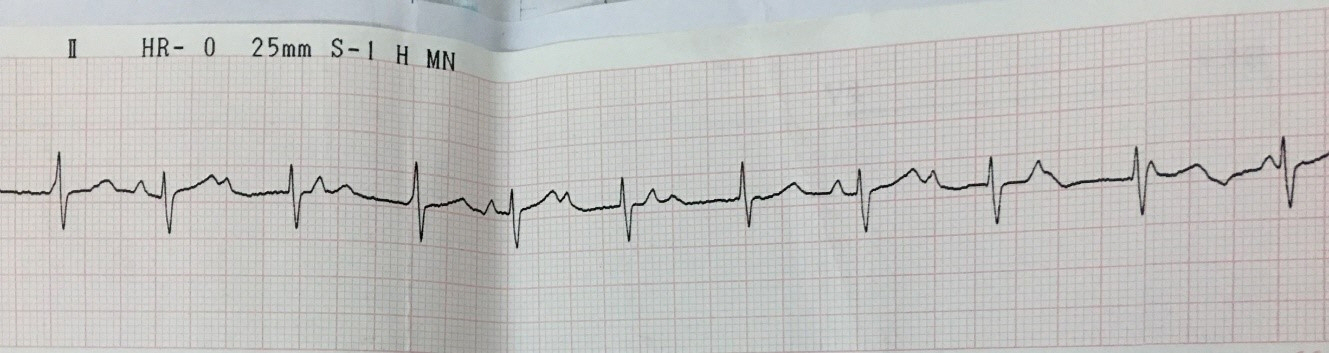
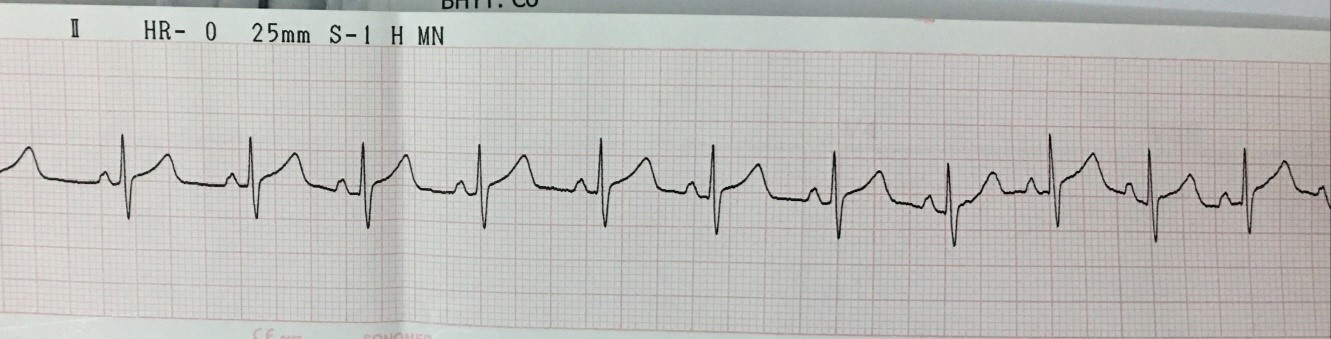



Case Summary
Complete heart block is a rare complication. In case it occurs, the following causes should be considered: (1) unsuitable selected patients for intervention;(2) technical issues;(3) oversized device;(4) deflection of the device;(5) abnormalities in the anatomical position of AV node and conduction system. In this case, the patient meets the criteria for transcatheter intervention, the morphology of ASD was "simple", the device was appropriate size and fixed well. Thus, abnormalities of the conduction system may be a probable reason to explain the complete heart block in this patient. And the question from this case is if we should release the device when transient heart block happened?


